You just flunked
"You
continue to post about raw oleander and try to relate it to properly prepared
oleander extract."
That has nothing to do with the reactions to oleander soup and teas - which
has killed people. By your own admission, oleander has 500 chemicals in
it. Cooking them does not change the fact that they are
"chemicals" which through human ingestion is chemotherapy and there is
no way in the world that you Tony can dismiss the fact that you are promoting
chemotherapy - the treatment you supposedly despise.
che·mo·ther·a·py 
(k


m

-th

r
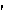
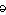
-p

,
k

m


-)
NOUN:
- The treatment of cancer using specific chemical agents or drugs that are
selectively destructive to malignant cells and tissues.
Another problem with oleander is that there has been very little, if any,
human trials. Through your blind (as in not getting feedback from all who
use it as you promote it) treatment you have no idea how many people have died
or become seriously ill/heart failures through ingestion of your recommendations.
That's one huge draw back to Internet diagnosis and treatment. It is well
known that an MD has to sign the death certificate and they have no way of
knowing what alternative therapies were involved in the treatment of the
patient.
Hulda Clark's zapper is better documented than your soup.
http://www.cancer.org/Treatment/TreatmentsandSideEffects/ComplementaryandAlte...
What is the evidence?
The effectiveness of oleander has not been proven. In test tube studies,
oleandrin, one of the substances found in oleander extracts, caused apoptosis (a
specific type of cell death) of prostate cancer cells. In other test tube
studies, Anvirzel appeared to slow the growth of human bladder cancer cells, but
human studies are needed to determine whether it will work in people. Very
early studies of carefully dosed Anvirzel in people with cancer have not yet
shown that it helps. Side effects included nausea and vomiting, aches, and
redness at the injection site, but the drug did not appear to affect the cancer
in these patients. One very early study of 18 patients with advanced cancer was
done primarily to determine the best dose of the drug. No measurable responses
were noted in patients’ cancer during this small study. Although there are
claims that Anvirzel improves quality of life, reduces pain, increases energy,
and causes cancer regression and remission, available scientific evidence does
not support these claims.
Another company had planned to offer an oleander extract that could be placed
under the tongue, which they named Xenavex. Phase I and Phase II clinical trials
on Xenavex were announced in 2005 on people with non–small-cell lung cancer.
However, the clinical trials were not done, and the announcements were later
removed from the federal clinical trials Web site. The company did not return
calls or e-mails about the product.
Before any form of oleander can be recommended for human use, it must be
thoroughly tested in people using the carefully controlled dosing and
observation procedures used in clinical trials.
Are there any possible problems or complications?
This substance may not have been thoroughly tested to find out how it
interacts with medicines, foods, herbs, or supplements. Even though some reports
of interactions and harmful effects may be published, full studies of
interactions and effects are not often available. Because of these limitations,
any information on ill effects and interactions below should be considered
incomplete.
The oleander plant is poisonous, and many people have died of heart failure
or respiratory paralysis after eating parts of the plant or drinking tea made
from it. Some of the symptoms and signs of oleander toxicity are nausea,
vomiting, colic, appetite loss, dizziness, drowsiness, high potassium levels,
dilated pupils, bloody diarrhea, seizures, loss of consciousness, slow or
irregular pulse, and heart block -- a blockage of the electrical impulses that
stimulate the heart to contract. There have been reports of death occurring
after oral and/or rectal administration of the extract from the plant. The
FDA has received reports of at least 2 deaths linked to Anvirzel.
Skin irritation from contact with oleander has occurred and allergies are
possible. One report observed that, when oleander was taken by a pregnant woman
12 hours before delivery, her baby was affected with seizures and a slowed heart
rate. No other cause for the seizures and low heart rate was found. This herb
should be avoided, especially by children and by women who are pregnant or
breast-feeding. Relying on this type of treatment alone and avoiding or delaying
conventional medical care for cancer may have serious health consequences.
 #136970
12 y
4,222
#136970
12 y
4,222
 #136970
12 y
3,942
#136970
12 y
3,942

