|
Getting mad or forgetful after taking a laxative? Well, according to the US Food and Drug Administration that’s exactly what certain best-selling laxatives can do to you.
Back in December of 2011, the FDA placed MiraLAX — a polyethylene glycol-containing blockbuster drug marketed by Merck & Co — on its Adverse Event Reporting System (AERS) in connection to “neuropsychiatric events.”

The MiraLAX-related posting on the FDA's website [1].
Besides MiraLAX, this warning also applies to Movicol, Dulcolax, Colyte, Colovage, Co-Lav, Clensz-Lyte, ClearLax, GoLYTELY, GaviLyte C, GlycoLax, Go-Evac, GlycoPrep, E-Z-Em Fortrans, Halflytely, Lax-a-Day, LaxLyte, MoviPrep, Macrogol, NuLytely, OCL, Peg-Lyte, Prep Lyte, Softlax, TriLyte, and all other brands with Polyethylene Glycol 3350 (PEG for short) as their active ingredient. The “3350” qualifier refers to the molecular weight of this particular variant of PEG.
Polyethylene glycol is made by stringing together molecules of ethylene glycol into a large polymer chain, hence the prefix poly-, Greek for many. On its own, ethylene glycol is used in automotive antifreeze and brake fluid. According to the National Institute for Occupational Safety and Health, it is an extremely toxic substance:
“Ethylene glycol is chemically broken down in the body into toxic compounds. It and its toxic byproducts first affect the central nervous system (CNS), then the heart, and finally the kidneys.Ingestion of sufficient amounts [as little as 30 ml — KM] can be fatal.” [2]
The term “neuropsychiatric events” in the FDA's safety alert refers to neurologic disorders of the central and peripheral nervous systems such as autism, dementia, depression, schizophrenia, multiple sclerosis, Alzheimer’s and Parkinson’s diseases, and similar others [3]. These conditions result from PEG's direct (through cellular damage) and indirect (through malnutrition of essential micronutrients) neurotoxicity. No surprise there considering the quotation above.
Lead, mercury, and arsenic are some of the best known neurotoxins. So are snake venom, curare, botulinum, and tetanus. PEG is more like lead or mercury — slow-acting, insidious, and difficult to pin down conclusively onto a variety of “slow-brewing” neurological disorders.
In addition to neurotoxicity, the following serious complications have been associated with polyethylene glycol-containing laxatives:
● Nephrotoxicity: PEG has been connected to nephrotoxicity, a euphemism for kidney damage [4] and it is counter-indicated for patients with kidney disease. This particular “side effect” is most likely related to the hydrolyzed (separated in water solution) molecules of ethylene glycol.
● Urticaria: PEG may cause allergy-related hives (urticaria) — raised red welts on the surface of the skin. Children are particularly susceptible to hives, and face a grave risk of anaphylaxis — a life-threatening allergic reaction that may develop within minutes or even seconds after ingesting a PEG-containing laxative. Links between PEG and urticaria have been documented as far back as 1991 [link].
● Esophageal perforations: Also known as Mallory-Weiss tear, esophageal perforations associated with polyethylene glycol electrolyte lavage solution have been reported as far back as 1991. These tears and related bleedings may occur in the mucus membrane of the lower part of the esophagus, or upper part of the stomach [link].
This particular side effect isn't directly related to MiraLAX which is taken in smaller doses, but the potential is always there, particularly among young children or patients with GI tract obstruction that may initially manifest itself as constipation.
All of the “collateral damage” from PEG shouldn't surprise anyone, least of all seasoned chemists, pharmacists, and medical doctors. This industrial chemical is manufactured by Dow Chemical Company for use in wood treatments, paints, coatings, rubber, textiles, detergents, and toilet bowl cleaners [4].

The cover page of the Carbowax product brochure by Dow Chemical Company, a leading manufacturer of PEG.
Technically, PEG is an osmotic laxative. Because of this property, it blocks the absorption of nutrients in the small intestine. Its extended use may result in severe malnutrition-related disorders, particularly in young children and older adults. Autism is one such disorder. It may take only two weeks of an acute iron or iodine deficiency to cause autism in a child younger than two.
The same properties of PEG that make it an excellent toilet bowl cleaner, also wipe clean the mucosal membrane of the large intestine, leaving the colon unprotected and cancer-prone, a situation similar to a dry mouth. On top of the mucosal membrane damage, a high osmotic gradient of polyethylene glycol solution decimates intestinal bacteria — single cell organisms — just as mercilessly as antibacterial soaps, antibiotics, or heavy metals.
The resulting dearth of intestinal bacteria is called dysbiosis in the United States, and disbacteriosis in the rest of the world. Dysbiosis reduces primary immunity, causes a broad range of neurological and blood disorders, makes occasional constipation chronic and more severe, ensures lifelong dependence on laxatives, and is behind ulcerative colitis, Crohn’s disease, and colorectal cancer.
When PEG is combined with a high fiber diet — a standard remedy for dysbiosis — problems double up because indigestible fiber tends to enlarge stools. In turn, large stools lead to habitual straining — a primary cause of enlarged hemorrhoids, anorectal nerve damage, anal fissures, diverticular disease, fecal obstruction, and genitourinary disorders such as rectocele — a protrusion of the rectovaginal wall inside the vagina.
A single recommended dose of MiraLAX contains 17 grams of pharmaceutical grade PEG powder [6], a humongous amount of what is otherwise an industrial-strength anti-fungicide, insecticide, and germicidestrong enough to preserve wood beams, railroad ties, and electrical poles from fungi, insects, and bacteria practically forever. It works by displacing water in wood, which makes it resistant to warping and rotting.
This is kind of ironic — the same people who will go out of their way to “eat organic” in order to avoid traces — we are talking micrograms — of fungicides, insecticides, and germicides in their foods, will then go on and ingest a heaping tablespoon of polyethylene glycol-containing laxative without blinking an eye. Or give it to their children...
Despite all of its well-established risks, MiraLAX has never been tested for safety in pregnant women and children:
“Safety and effectiveness [of PEG] in pediatric patients has not been established.” “Animal reproductive studies have not been performed with Polyethylene Glycol 3350 NF. It is also not known whether Polyethylene Glycol 3350 NF can cause fetal harm when administered to a pregnant woman, or can affect reproductive capacity...” [7]
For these and other prudent reasons MiraLAX was approved by the Food and Drug Administration back in 1999 only for use by adults, and for no longer than 7 days. In spite of this clear and unambiguous rule, pediatricians routinely prescribe PEG-containing laxatives to children of all ages [8] anyway. Equally disturbing, many doctors encourage adults and children alike to take them indefinitely, even though the label clearly states: “Use no more than 7 days.”
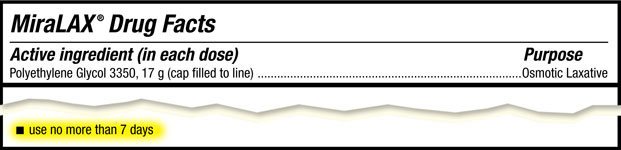
The cutout from the MiraLAX product label. [9]
A citizen petition “To Investigate Polyethylene Glycol 3350 Product Safety for Use with Pediatric Patients” was filed with the U.S. Federal Drug Administration on June 6, 2012. Here is what it said:
“As of March 2012, the FDA Adverse Event Reporting System showed 2257 reported adverse events related (in any way) to PEG products - up from 7 in 2001. Included in the adverse events reported are serious kidney, urinary, bowel, blood, skin, and neuropsychiatric symptoms - and at least 3 children's deaths. [PDF link]"
Keep in mind that only a tiny percentage — around 1% — of serious side effects ever get reported to the FDA [link]. It's probably even less with MiraLAX and its clones — unlike with prescription drugs, making direct connections with PEG side effects is far more challenging because so many of them aren't immediate; or because nobody expects to encounter severe complications from a “mere” over-the-counter laxative; or because small children can't communicate their sufferings in words; or because it's so hard to identify whodunit among adults who are already taking a daily cocktail of other potent drugs; or because these outcomes are falsely blamed on genetics, environment, and old age.
The use of laxatives is particularly common throughout pregnancy. It’s entirely possible that ethylene glycol molecules penetrate the placenta and cause neurological damage in fetuses. Infants may be equally affected via breast milk, or milk supply may be poor quality or too low. And that's on top of the maternal malnutrition and neurological damages I've described above.
Notably, MiraLAX was introduced back in 1999 [10], about the same time the epidemic of autism began sweeping across the United States in earnest. It doesn’t take a PhD in epidemiology to infer a possible connection between these two happenings.
If anyone you know is pregnant or nursing, please let them know about the possible role of polyethylene glycol-containing laxatives in autism, postpartum depression, and other neurologic disorders. The connection between these conditions and MiraLAX has never been proven conclusively because conducting this kind of clinical research is illegal. Still, as a lawyer might say, “the preponderance of the evidence is overwhelming,” so you are better off being safe now than sorry later, particularly with something as easily avoidable as PEG-containing laxatives.
Laxatives with polyethylene glycol are also a potential menace to seniors who rely on them to alleviate medication-related constipation — a common side effect of taking multiple prescription drugs for depression, insomnia, and hypertension.
Please forward a link to this page to your parents and grandparents, so they can protect themselves from memory loss, dementia, and Alzheimer’s disease made eerily possible by taking polyethylene glycol-containing laxatives.
Unfortunately, despite all of the above issues and the FDA’s published warning related to polyethylene glycol safety, you still won’t find a hint of concern in MirLAX’s patient literature. All it says: “MiraLAX® is safe and effective – and available without a prescription [11].”

No comments...
How can Merck get away claiming that MiraLAX is “safe” and “without harsh side effects?” Well, just like it did with Vioxx, another presumably “safe” miracle drug [12]. Following this well established pattern, Merck will continue milking MiraLAX's franchise for as long as it can. When the time will finally arrive to recall it, Merck's army of lawyers will keep fighting its victims until they'll settle en masse for pennies on the dollar [13]. It's a cost of doing business, nothing personal...
But this ugly story doesn’t end with laxatives. It actually gets even uglier — food grade polyethylene glycol (under the Carbowax Sentry trade name) is commonly added to chewing gums, table-top sweeteners, and energy drinks. It is also used as a base in solid and liquid soaps, shampoos, bath and shower gels; body and face creams and lotions; toothpastes, ointments and suppositories; as a tablet binder in drugs and supplements; as a lubricant in vaginal gels and eye drops; and as a solvent in cough medicines and elixirs. Many of those products are used by children of all ages [14].

Illustrations from the Carbowax Sentry product brochure by Dow Chemical Company. Deadly effective, I must say...
Please alert your family, friends, and colleagues with young children about the dangers of polyethylene glycol in their foods and medicines via your Facebook page, Twitter feed, e-mail, website, or blog.
To wean yourself, your spouse, your children, or your parents from PEG-containing laxatives, begin from reading this page: How to Overcome Chronic and Intermittent Constipation. Your mileage may vary depending on your age and degree of damage, but unlike with MiraLAX and its clones, you certainly won't go crazy from trying.
Author's note
If you think this article is a tempest in a teapot, you have no idea just how insidiously damaging constipation may be to someone's health, physical and mental alike. And even more so to a hysterically crying baby with zero tolerance for pain, but no capacity to describe in words what's hurting her.
Adding insult to injury, after getting nuked with an abdominal CT scan to “diagnose” constipation and, then hooked on MiraLAX by the very doctors expected to protect her health, she may end up autistic and cancer-prone for the rest of her life.
All that said, I am not yelling “fire” here for the sake of making a point. To the contrary — this site provides safe, effective, and natural solutions for resolving the nastiest kinds of “constipations,” and I hope you'll use them.
If you are interested in exploring connections between autism and nutritional deficiencies related to PEG, please check out these articles:
Sullivan KM. Iodine deficiency as a cause of autism. J Neurol Sci. 2009;276(1-2):202; author reply 203 [link].
Hergüner S, Keleşoğlu FM, Tanıdır C, Cöpür M. Ferritin and iron levels in children with autistic disorder. Eur J Pediatr. 2012 Jan;171(1):1436 [link].
Even though dysbiosis — the dearth of intestinal microflora in the large intestine — isn't recognized in the United States as a medical condition, it doesn't mean that there isn't plenty of academic research to support its role in countless pathologies:
Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012 Aug;4(8):1095-119 [link].
Zaffanello M, Zamboni G, Fontana E, Zoccante L, Tatò L.; A case of partial biotinidase deficiency associated with autism. Child Neuropsychol. 2003 Sep;9(3):184-8 [link].
Vitamin K deficiency, The Merck Manual of Diagnosis and Therapy [link].
Dieu-Thu Nguyen-Khoa, MD; Vitamin K Deficiency; Etiology; Medscape Reference [link].
If push comes to shove, don't fight your doctors over this contentious topic — just refer them to these links. These references are as mainstream as it gets. Incidentally, the “Merck” in the Merck Manual is the same “Merck” as in MiraLAX story. Go figure...
References
Click the [link] symbol to view a related web page. Click the Backspace key to return to the original location.
1. U.S. Food and Drug Administration, “Polyethylene Glycol (PEG) 3350 over-the-counter oral laxative (Miralax),” Potential Signals of Serious Risks/New Safety Information Identified by the Adverse Event Reporting System (AERS) between October - December 2011, last modified October 18, 2012, [link].
2. National Institute for Occupational Safety and Health (NIOSH), "ETHYLENE GLYCOL : Systemic Agent,"The Emergency Response Safety and Health Database, last reviewed May 12, 2011, accessed January 16, 2013, [link].
3. The Merck Manual for Healthcare Professionals, "Neurologic Disorders," Accessed January 16, 2013, [link].
4. F. Alan Anderson, "Special Report: Reproductive and Developmental Toxicity of Ethylene Glycol and Its Ethers," International Journal of Toxicology, 18, no. 2 (1999): 53-67, [link].
5. Dow Chemical Company, "CARBOWAX™ Polyethylene Glycols: Innovation, Performance, Flexibility and Quality from the Global Leader in PEGs," published October 2011, [PDF file].
6. “MiraLAX easily dissolves in four to eight ounces of water, fruit juice, or any other beverage. And each 7, 14, and 30 dose bottle top is a measuring device that indicates one 17g dose.” –MSD Consumer Care, Inc., “How do I take MiraLAX?,” FAQs, [link].
7. Breckenridge Pharmaceutical Company, “Polyethylene Glycol,” Drugs.com, last revised January 2012, [link].
8. Catherine Saint Louis, “Drug for Adults is Popular as Children’s Remedy,” New York Times, May 25, 2012, [link].
9. “Stop use and ask a doctor if you need to use a laxative for longer than 1 week,” MSD Consumer Care, Inc., “Directions,” “Warnings,” MiraLAX product label, [PDF file].
10. “In 1999, when the F.D.A. first approved MiraLAX, the patient materials included the warning: “MiraLAX should not be used by children.” – Catherine Saint Louis, “Drug for Adults is Popular as Children’s Remedy,” New York Times, May 25, 2012, [link].
11. Consumer Care, Inc., “What is MiraLAX?,” MiraLAX Product Brochure, page 2, [PDF file].
12. “Up to 140,000 extra heart attacks may have been caused in the US by the recently withdrawn drug Vioxx since its launch in 1999.” –Shaoni Bhattacharya, “Up to 140,000 heart attacks linked to Vioxx,” NewScientist.com, published January 25, 2005, [link].
13. “Merck settled in late 2007 for a relative pittance, resolving some 50,000 Vioxx cases for just under $5 billion. It was a far cry from the $25 billion to $50 billion in liability that analysts had predicted when Merck withdrew the drug.” –Snigdha Prakash, “The Cover-Up Artist: Why is the CEO of Merck leading the sex-abuse investigation at Penn State?,” Slate.com, posted November 15, 2011, [link].
14. Dow Chemical Company, CARBOWAX™ SENTRY™ Polyethylene Glycols: Innovation, Performance, Flexibility and Compliance from the Global Leader in PEGs,” published October 2011, [[PDF file].
All references above were compiled in December of 2012. If any of the links is missing, you can locate it on theInternet Archive website.
|








January 29, 2013 by Staff
Science