|
The Acetaldehyde Game
Explores the interaction of acetaldehyde with folic acid, cobalamin, and urea.
Date: 10/22/2012 7:28:15 AM ( 13 y ) ... viewed 24512 times Let's play a combination game of "pictionary" and "find Waldo" to see if we can transfer the information gained from the side-trip into industrial acetaldehyde scavenging to good use in a medical context.
• See "Acetaldehyde + Industrial Contaminant" http://curezone.com/blogs/fm.asp?i=1995773
In this game we'll be looking for molecular group similarities (the pictionary part) that might provide attractive binding sites for acetaldehyde (Waldo).
Although folic acid (vitamin B9) is an essential dietary requirement of everyone, it is particularly important during pregnancy where deficiencies are linked to neural tube defects involving the brain and spinal cord [1].
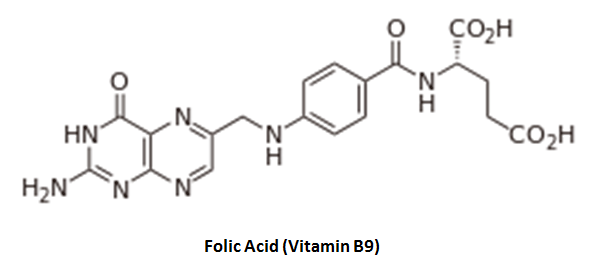
A prominent and recurrent feature of the industrial acetaldehyde scavengers examined previously is the presence of the amino group (-NH2) where the nitrogen provides an attractive binding site (e. g. 1,8-diaminonaphthalene).
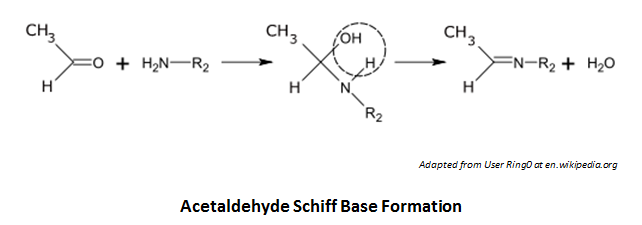
Does folic acid have an exposed amine group that might be vulnerable to interaction with acetaldehyde? Indeed, acetaldehyde may form a Schiff base (C=N) with the trailing amine (without cyclization in this case):
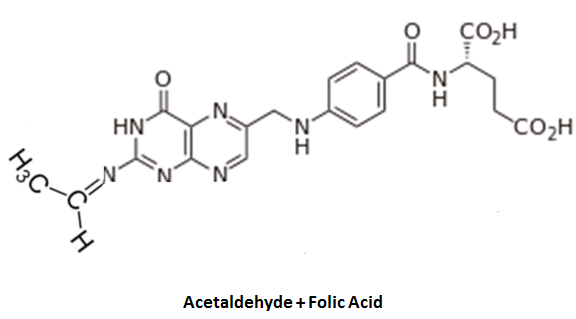
"Waldo" (aka acetaldehyde) has attached itself to the end of the folic acid molecule:
Acetaldehyde has been caught interfering with the enzymatic cofactor roles of vitamins before in the case of pyridoxal (vitamin B6) where it has the potential for forming a Schiff base with the lysine residue amino attachment point for the vitamin in several different enzymes:
• See "Acetaldehyde + Pyridoxal" http://curezone.com/forums/fm.asp?i=1982110
This time, by forming a Schiff base with folic acid itself, it can also interfere with the critical functions of this vitamin. If acetaldehyde gums up either the structure of an enzyme or the structure of a critical substrate that is supposed to fit into a reaction pocket, then the result is the same: a dysfunctional biochemical pathway. Since the body uses folate to synthesize DNA, repair DNA, and methylate DNA, this provides yet another link from acetaldehyde exposure to disregulation of cellular maintenance and ultimately to cancer [2]:
• See "Acetaldehyde + Cancer" http://curezone.com/forums/fm.asp?i=1951252
Even when dietary or supplementary folate intake is adequate, a high commensal yeast load releasing acetaldehyde may result in a functional folate deficiency. As well as fetal defects and cancer, this could lead to glossitis (inflammation of the tongue), diarrhea, depression, confusion, and anemia. Folate deficiency that manifests even when classical measurement of blood concentrations, peripheral blood, and bone marrow examinations appear to be normal is recognized in alcoholic patients where high acetaldehyde levels are associated with the incomplete metabolism of ethanol [3]. When acetaldehyde is metabolized by xanthine oxidase (a molybdenum-based enzyme) it can generate the superoxide anion reactive oxygen species capable of cleaving folate [4].
• See "Acetaldehyde + Reactive Oxygen Species" http://curezone.com/forums/fm.asp?i=1959622
Since folic acid and cobalamin are closely coupled, both in their functionality and deficiency symptoms, consider the configuration of the largest and most structurally complex vitamin:
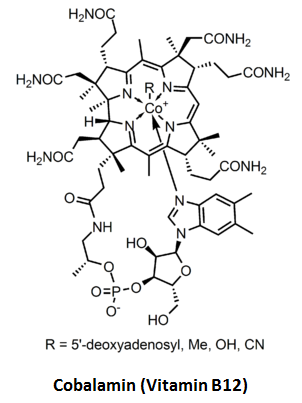
Note that there are six amide side groups (-CONH2) surrounding a cobalt-containing ring. This amide group also appears in the list of industrial acetaldehyde scavengers (e.g. salicylamide and malonamide). One of the simplest amides, acetamide:
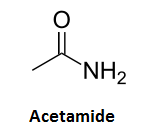
reacts with acetaldehyde to form ethylidene-N,N-diacetamide [5]:
CH3CHO + 2 CH3CONH2 --> (CH3CONH)2CHCH3 + H2O

This suggests that any one or all or a combination of the amide side chains of cobalamin (like red-wigglers angling for bass) could become targets of acetaldehyde attack with loss of vitamin B12 structure and activity potential. Cobalamin plays a role in brain and nervous system functioning, fatty acid synthesis and energy production, and like folate is involved in DNA synthesis and regulation. Early biomarkers of B12 deficiency include teeth clenching and myoclonic jerking including tics, twitches, and "sleep starts". No fungi, plant, nor animal can synthesize this vitamin, making it particularly vulnerable to deficiency in restrictive or non-supplemented diets that avoid foods containing B12 from a bacterial source.
By tapping into the energy field involved in the absorption, transport, and delivery of nutrients in the body, an OPK (Orthomolecular Psychokinesiology) test with a kinesiology hand grip can tell you non-invasively in a few seconds whether either B9 or B12 or both are deficient [6].
Whether we have realized it or not; yeast, acetaldehyde and disease have been enmeshed for eons. This means that a host of somewhat unorthodox remedial techniques may have arisen by trial and error for dealing with this conceptual triad.
Urophagia is the consumption of urine and urine therapy has been used by many cultures throughout history for medicinal purposes. All amino acids are nitrogen-containing substances. Numerous pathways in the body lead to the urea cycle which produces urea as a disposal technique for excess nitrogen and ammonia (NH3) that can raise cellular pH values to toxic levels. Urea is a symmetrical amide:
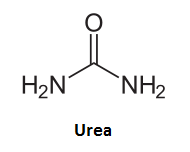
Our game's pictionary skills might suggest that this substance would also be a good candidate for reaction with acetaldehyde -- this is true. Acetaldehyde will react with both amine groups of urea to form ethylidenediurea [7] where "Waldo" is now sandwiched between two urea molecules and has lost its aldehyde reactivity.
CH3CHO + 2 NH2CONH2 <--> NH2CONH-CH(CH3)-NHCONH2 + H2O
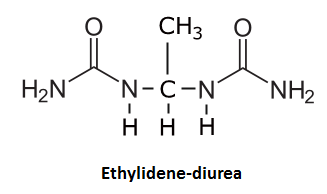
Although the ingestion of urine may provide some relief from yeast-released acetaldehyde via the ethylidenediurea reaction, there are numerous negative aspects associated with this practice. Considering that the body has carefully accumulated and solubilized the contents of urine for removal, putting these waste byproducts back into the system may not only upset the body's hydration balance but also reintroduce toxins and pathogens that should have been excreted.
However, the message from MMS:
• See "Acetaldehyde + MMS" http://curezone.com/forums/fm.asp?i=1910030
and hydrogen peroxide:
• See "Acetaldehyde + Hydrogen Peroxide" http://curezone.com/forums/fm.asp?i=1985227
and dirt:
• See "Acetaldehyde + Dirt For Dessert" http://curezone.org/blogs/fm.asp?i=1991592
and now urea is that the healing aspects of these potentially toxic substances could be attributed to their interactions with yeast-released acetaldehyde and that there are better ways of dealing with the problem [8].
[1] Pitkin RM, "Folate and neural tube defects.", Am J Clin Nutr. 2007 Jan;85(1):285S-288S.
http://www.ncbi.nlm.nih.gov/pubmed/17209211
[2] Homann N et al., "Microbially produced acetaldehyde from ethanol may increase the risk of colon cancer via folate deficiency.", Int J Cancer. 2000 Apr 15;86(2):169-73.
http://www.ncbi.nlm.nih.gov/pubmed/10738242
[3] Gimsing P et al., "Vitamin B-12 and folate function in chronic alcoholic men with peripheral neuropathy and encephalopathy.", J Nutr. 1989 Mar;119(3):416-24.
http://www.ncbi.nlm.nih.gov/pubmed/2537891
[4] Shaw S et al., "Cleavage of folates during ethanol metabolism. Role of acetaldehyde/xanthine oxidase-generated superoxide.", Biochem J. 1989 Jan 1;257(1):277-80.
http://www.ncbi.nlm.nih.gov/pubmed/2537625
[5] Noyes et al., "Aldehyde—Amide Condensation. I. Reactions between Aldehydes and Acetamide", J. Am. Chem. Soc., 1933, 55 (8), pp 3493–3494
http://pubs.acs.org/doi/abs/10.1021/ja01335a085
[6] "Deficient Or What?" and "Pseudo-Deficiencies" in "Astrophysiology… and Yeast", 2011.
http://www.scribd.com/doc/74090699
http://www.epubbud.com/book.php?g=7JQU45V8
[7] Ogata et al., "Kinetics of the Condensation of Urea with Acetaldehyde", J. Org. Chem., 1965, 30 (5), pp 1636–1639
http://pubs.acs.org/doi/abs/10.1021/jo01016a076
[8] "Wondro -- Inside Out", 2012.
http://www.scribd.com/doc/101099776
http://www.epubbud.com/book.php?g=7D42SJH6
Add This Entry To Your CureZone Favorites! Print this page
Email this page
 Alert Webmaster Alert Webmaster
|







