Vaccine manufacturer’s documents sho...
Vaccine manufacturer’s documents sho...  befurther
12 y
10,015
RN Warning
† Cross †
befurther
12 y
10,015
RN Warning
† Cross †
Extraordinary Parenting
The Ultimate Guide to Modern Parenting Ebook
Energy Awareness Course
Use CureZone kode to get a free session!
J.Crow’s® Lugol’s Iodine
Free S&H.Restore lost reserves.J.CROW’S®Lugol’s Iodine Solut...
Original Hulda Clark
Hulda Clark Cleanses
Advertisement
Try Moringa Today!
Moringa isn’t just a superfood; it’s a smart tool in our quest for global health!
Much the same as in US, UK, Australia and other countries, Japanese obstetricians and gynecologists advocated HPV vaccines as a highly effective method of preventing uterine cervical cancer. In 2008, they formed an organization named “the Congress of Professionals for Suppressing Uterine Cervical Cancer” to further promote this recommendation.
HPV vaccination programs began in 2010 under a recommendation made by the Japanese Ministry of Health, Labor and Welfare (JMHLW) to administer HPV vaccines to girls from 11 to 14 years old. The Japanese government allocated 15 billion yen (187.5 million dollars) for urgent HPV Vaccination programs. As HPV vaccination was voluntary and not yet mandatory, local governments eagerly recommended the vaccination. Officials visited junior high schools to advocate the effectiveness of the vaccine and persuade students to be vaccinated. They also stressed that the expensive vaccination (48,000 yen, $600, for three shots) would be free within the 2 year limit. Municipal offices sent letters to families which had girls of the targeted age group to urge vaccination.
Japan Medical Association and Japan Pediatrics Association supported HPV vaccination program. From the end of 2012 to Feb. 15 2013, JMA conducted a signature-collecting campaign for a petition to urge the addition of seven vaccinations (HB, PCV7, PCV23, Hib, HPV, chicken pox, mumps) to the list of mandatory ones.
After discussions in the Japanese parliaments, it was decided to add three vaccinations (HPV, Hib and PCV7) to the mandatory ones on Mar 28 2013. There was no political party which stated an opinion opposing the addition of HPV vaccines.
March 11 2011, huge earthquakes and tsunami attacked the north-eastern area of Honshu island of Japan, and atomic power plant in Fukushima lost external power supply and lost the means to cool reactor cores.
Aftershocks hit repeatedly. The biggest one was during the night of April 7. Nuclear power plants in Fukushima blew up by hydrogen explosion on Mar 12 and 14.
In the midst of this turmoil, a non-commercial video made by Advertising Council Japan was broadcasted on TV repeatedly and repeatedly. In that video, an actress known to have recovered from uterine cervical cancer and her daughter stressed the importance of cervical cancer checkups. Two or three months later, this video program was replaced with HPV vaccine promotion video programs of Cervarix (GSK K.K.) and Gardasil (MSD K.K.).
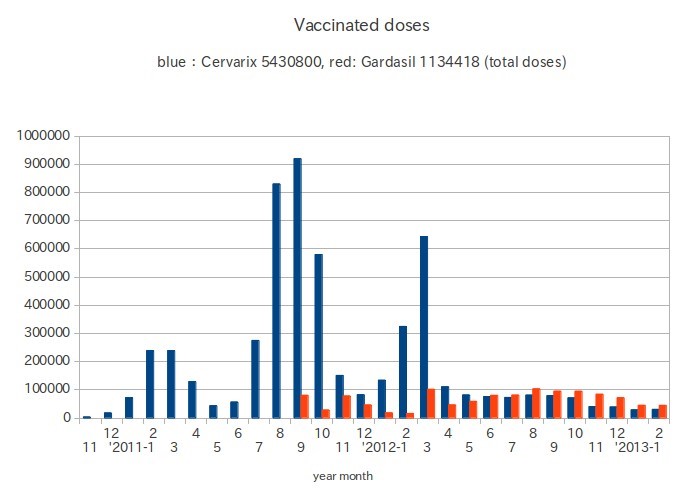
Up to today, 3.28 million girls, including adult women, were vaccinated with HPV vaccine. Total dose is estimated as 8.64 million. The figure below shows the transition of vaccination count reported to JMLHW from local governments. The population of Japanese girls age 11 is, for example, 580,000. March is the last month of the academic year.Joint meetings of the Vaccine Adverse Reactions Review Committee (JVARRC) are held three times a year. 1968 cases of adverse events have been reported to JMLHW and JVARRC. 358 cases which were evaluated as serious by the committee and JMLHW are included.
The presence of girls suffering from adverse effects of cervical cancer vaccine was revealed gradually from the beginning of 2013. The Nationwide Liaison Association of Cervical Cancer Vaccine Victims and Parents was organized by efforts of Toshie Ikeda, Mika Matsufuji and members of municipal assembly on Mar 25, 2013.
Parents of vaccine victims called our health minister on April 8. During the press conference after that, videos in which girls are suffering from walking disturbances, tic of the body, absence seizure and choreic movement was presented.
June 14 2013, JVRRC decided to suspend the recommendation for HPV vaccination. The same day, JMLHW sent a notification to local governments that HPV vaccination should not be recommended actively until the time when MHLW and JVRRC made a report as to the safety concerns of HPV vaccines and that vaccination should be done for those who want vaccination. In the meantime, HPV vaccination should be available for those who wished it.
 The Congress of Professionals for Suppressing Uterine Cervical Cancer and Japan Pediatrics Association made a statement that JMHLW should “withdraw the suspension of recommendation” for HPV vaccination and should “restore the recommendation” for the vaccination.
The Congress of Professionals for Suppressing Uterine Cervical Cancer and Japan Pediatrics Association made a statement that JMHLW should “withdraw the suspension of recommendation” for HPV vaccination and should “restore the recommendation” for the vaccination.
Does vaso-vagal reflex cause consciousness derangement and falls?
In the early period of HPV vaccination program, many girls who were injected with the vaccine fell down to the floor within several minutes injuring their heads or faces. Some girls fractured their jaws and teeth. JMHLW and the JVRRAC announced that the reaction was due to vaso-vagal reflex and vaccinated girls should be kept in medical facilities and lying down for at least 30 minutes.
Vaccine manufacturers have an obligation of post-licensure vigilance. A document titled “On the issue of adverse reaction in relation to syncope after vaccination” had been published on every committee meeting where additional cases were presented. The document consists of two parts: each one is made and submitted by GSK K.K. and MSD K.K. The one dated Mar 25 2012, consisting of 77 pages, is estimated as the best. So, following article is based on this report.(http://www.mhlw.go.jp/stf/shingi/2r9852000002c06s-att/2r9852000002c0cw.pdf)
381 cases in which syncope and/or falls occurred after injection were collected and analyzed by GSK. MSD analyzed 91 cases.
Severe pain is thought to bring vaso-vagal reflex. Vaso-vagal reflex brings hypotension and bradycardia (low blood pressure less than 80 mmHg and low heart rate less than 30~40/min. Numerical values are author’s estimations). Low blood pressure results in an insufficient blood supply to the brain and normal brain function cannot be maintained.
But, with my experience, visual stimuli such like seeing wounds or blood may bring nausea and hypotension. Pain does not always cause vaso-vagal reflex.
Table 2 made by GSK K.K. (page4) and that made by MSD K.K. (page 59) shows the states or conditions of the recipients while unconscious.
Items and legends of the tables are translated word for word.
Cervarix: page 4
Table 2: Situations or states of the cases who lost consciousness (cases in whom symptom occurred within 30 min)
Consciousness deranged: YesNoUnknown or not writtenNo. of casesRatio %No. of casesRatio %No. of casesRatio %Hypotension: existed or not?7820.54912.924366.6Clonic motion (convulsion included): existed or not?7219.818749.112232Secondary injuries like fall *1: existed or not?381023561.710928.3Treatment such as drip infusion or oxygen inhalation was necessary.10126.519952.28121.3Risk factors prone to syncope (past histories, complications) :exited or not5213.632986.4--Stress or anxiety at injection: existed or not?15139.67519.715540.7*1: more precise explanation of secondary injuries: bruises mainly at head, chin, face and back (their postures before collapsing were sitting in most cases) Others: nasal bone fracture (sitting), hematomas at the head bruise (standing), mild brain concussion shown by MRI (standing), laceration of the skin of face and suture was needed (standing), bruise and laceration of chin with fracture of teeth (sitting)Gardasil: page 59Table2: Situations on losing consciousness and states of the casesConsciousness deranged: YesNoUnknown or not writtenHypotension : existed or not29 (32%)28 (31%)34 (37%)Clonic motion (convulsion included): existed or not23 (25%)66 (73%)2 (2%)Secondary injuries like fall *1: existed or not13 (14%)78 (86%)0 (0%)Treatment such as drip infusion or oxygen inhalation was necessary26 (29%)63 (69%)2 (2%)Risk factors prone to syncope (past histories, complications): exited or not12 (13%)63 (69%)16 (18%)Stress or anxiety at injection: existed or not35 (38%)41 (45%)15 (16%)
*1: precise overview of secondary injuries: in most cases bruises are at head, chin and face. Postures before collapsing were sitting in 8 cases, standing in 4 cases, unknown in 1 case. In 7 cases who had injured in the chin and face, loosing/fracture/dislocation of teeth (3 cases), fracture of mental bone (1 case), bruise in mental bone and lip (6 cases), nasal bleeding (1 case).
In the case of Cervarix, the first row in the above table shows that low blood pressure was observed only in 39 cases (20%). The second row of the table is written as “Clonic motion (seizure is included) 18.9%”. Clonic motion is an involuntary motion in which arms and/or legs are jerked and extended rhythmically, i.e. seizure.
The first and the second row of table 4 shows that girls did not necessarily fall down with hypotension. Injection of Cervarix itself seems to induce convulsions in the vaccinated girls. It is the same in the case of Gardasil.
GSK had made a file titled as “List of cases with secondary injury to syncope brought by injection of Cervarix” in which 41 case records are contained (page 7 to 35).
Seizure or convulsion occurred in 12 cases (case 1, 3, 8, 9, 10, 17, 18, 19, 24, 26, 37 and 39).
In case 26 (see Addendum), tonic-clonic seizure and apnea occurred just after the injection, although blood pressure was not low. Case 15 was also with apnea.
These tables and case records in this document may show that girls fell to the floor with an attack of unconsciousness brought by hypotension (pre-shock). But these also show that girls had been attacked with seizures, which caused unconsciousness and loss of controls of skeletal muscles of the body. As a result, they fell to the floor like a pole. As girls could not prepare a defensive posture on falling, they injured by hitting their heads and jaws.
Consciousness derangement occurred more than 30 minutes after injection in vaccinated girls.
Cervarix: page 5
Table 4: Cases in which unconsciousness occurred more than 30 min after vaccination.within 24 hoursover1 dayWithin 24h but time unknownTime unknown<1 h<2 h<3 h<4 h<18h<24h>1 day>2 day>3 day>6 day>7 day>23 day21111113431114520
In 7 vaccinated girls, consciousness derangement occurred within 24 hours. In 23 vaccinated girls, it occurred from 24 hours to 7 days. The sum of the raw is 95. In these girls, it occurred more than 30 min outside medical institutions. In these girls, syncope or unconsciousness occurred outside medical institutions.
If recipients fell into vaso-vagal reflex and pre-shock, they might recover within 30 minutes at the most. Therefore, the cause of consciousness derangement or convulsion was not brought with hypotension due to vaso-vagal reflex.
GSK made a list of cases with late syncope attack which occurred more than 30 min after injection of Cervarix, and which title is “Cases in whom syncope occurred more than 30 min after injection of Cervarix” (page 36 to 52).
In some cases, diarrhea, menstrual cramps, taking bath or generalized urticarial seemed to bring syncope (case 1, 5, 7, 9, 11, 14, 23 and 28). But syncope is unusual in healthy teenager girls.
Convulsion or seizure occurred in 8 cases (case 2, 3, 8, 13, 16, 18, 24 and 30).
It should be considered that some brain damage occurred in these cases.
(In case 19, on 6th day after injection, cardiac and pulmonary arrest occurred after 100m running of a button relay in training for a sports festival of the junior high school. CPR was successful by ambulance team at school and by doctors in a hospital. But severe brain damage occurred. She went on to an artificial respirator for one week).
As to Gardasil, table 4 and list are absent.
(End):
[Addendum]
In the above document, three lists of case records are included. As to Cervarix, two lists are included: one is a list in which 41 case records are contained and titled as “List of cases with secondary injury to syncope brought by injection of Cervarix” (page 7 to 35) and another is a list in which 30 case records are contained and titled as “Cases in whom syncope occurred more than 30 min after injection of Cervarix” (page 36 to 52).
As to Gardasil, one list is included, in which 13 case records are contained and titled “List of cases in whom secondary injury, such like fall, occurred with syncope after injection of Gardasil” (page 61 to 74).
Case record of secondary injury after administration of HPV vaccine (Cervarix)
Case 24, page 25
Case record of secondary injury after administration of HPV vaccine (Gardasil)Teen aged girl.Previous history: hypotension2011/07/11 16:38Administration of CervarixLot No.: AHPVA129CAAdministration point: left upper arm muscleTime: first2011/07/11 16:43After administration, she walked a few steps from treatment room and fell down by facing upward with syncope and with tonic seizure (incontinence, BD 82/50). She regained consciousness within a few minutes.As she had hit the back of her head, she consulted with neurosurgery. No abnormality was found.Position before fall: standingActivity: after administration (about 5 min)Preceding signs or alarm: noGradual onset or sudden onset: sudden onsetManner of fall: fall down by facing upwardSkin color: paleDuration of unconsciousness: several ten sec.Biting of tongue: noComplete unconsciousness: yesTake any drug: noPhenomenon recovered with supine position or caudal position? : yesVital signs: during fall BD 80/50, after recovery 92/52 (mmHg)After regained consciousness, anything had happened: yes, incontinence and sweating.Could she recall anything about her unconsciousness: noPast history of syncope: noSpecial examination: noRecurrence: noAny medication: noCategory of anaphylactic shock 5 category: category (5)
Case 1; Gardasil, page 61
Case record in which unconsciousness occurred more than 30 min (3 and 30 days) after injection of CervarixCase 1: teen aged girl, date of administration 2011/9/2Administration of Gardasil: Needle had replaced with GA (25G/1”) which was took down from Nipro syringe. About 2 minutes later, when she was sitting on a sofa in the waiting room of the clinic, she fell on her face unconsciously (duration 2, 3 second) and hit her forehead.Body temperature before administration: 36.7CPosture before attack: sittingPrevious history of losing consciousness: noCause of fall: vaso-vagal reflexAbnormalities in vital signs and electrolyte: noBlood Pressure: 80/55 mmHg, Heart rate: 60Blood glucose, ammonia, ethanol: not examined, no values(Doctor’s) Comment: She did not seem to have mental strain before and during administration. She did not complain of pain. Asked after regained consciousness, she was smiling just before fall and she had no previous history of discomfort on administrations of other vaccines or collecting blood.
Case 13: Cervarix, page43
Case 13: teen aged girl, administration date 2011/08/18, Cervarix2011/08/18 body temperature before administration: 36.7C, blood pressure 110/802011/08/18 11:25 inoculation of Cervarix in a clinicLot No.AHPVA143CAInjected point: deltoid muscle (left arm)Times of administration: firstClinically, no abnormality was found.2011/08/21 11:30She woke up and took breakfast/ lunch.While mom took shower, her daughter operated PC sitting on a chair. It took 1 or 2 min. Mom found her daughter falling to the floor with chair.She closed her mouth firmly and did not respond on calling her name. Her face was pale and breathing had stopped. In order to revive, mom tried to open her daughter’s mouth by inserting mom’s fingers in her daughter’s mouth. But her daughter bit mom’s fingers strongly, mom could not pull out the fingers for 5 minutes. As her daughter loosened biting, mom became free and could call ambulance. Her breathing was shallow and cyanosis was found. She regained consciousness 5 to 10 min later for sake of mom’s nursing. She closed her mouth firmly but no clonic motion was found in her arms or legs. She could walk and get into an ambulance car. She was transferred to hospital and admittedAs she was heard afterward, she could not recall anything.She was transferred to hospital and admitted.2011/08/21-2011/08/25She was hospitalized for examinations. Brain CT, MRI, EEG (for 3 days) showed no abnormality at neurology.2011/09/17-2011/09/18She took part in two-days private school.2011/09/18 6:00She woke up.2011/09/18 6:30She was attending the class before breakfast.She attended the class and was sitting on a chair. She fell into unconsciousness. She fell down to the left and onto the floor. She seemed to pick up something. No clonic motion was observed and she breathed. She was unconscious. She was laid flat on the floor and regained consciousness 5-10 min later. She could stand up and walk with aid. She sat on a chair outside the classroom and her head was cooled with ice bag on her forehead. 5-10 min later, she returned to the classroom and continued studying.While her forehead was being cooled, her consciousness regained. But she could not recall falling and her forehead being cooled with ice bag.2011/09/18 7:30Received a phone call from the second school, mom went to pick up her. She slept deeply in a train.2011/09/20She was checked at neurosurgery. Brain CT showed no hematoma.There are bruises around right eye socket, right forehead, right temporal and right chin. She said there were petechiae on her right shoulder.She complained of being sleepy at present. Her heart rate was slow.Date unknownAs EEG, took at neurosurgery of the other hospital, showed spikes and it indicated epilepsy, she was medicated with anti-convulsant.As she did not visit to my clinic after the second injection, I consider that following up this case is difficult.This article first appeared at SaneVax Inc.
More
- HPV Vaccines: A Human Rights Violati... befurther
12 y
7,722
This is a reply to # 2,110,517
By K Paul Stoller, MD*, FACHM
Cervical cancer, the second-most common cancer in young women, is particularly prone to be found in the down trodden and in impoverished countries. But this is no endorsement for Human Papilloma Virus (HPV) vaccines. In fact this is about revealing that HPV vaccines were created for only one reason and it wasn’t as a humanitarian effort to minimize cervical cancer. It was created by greed to create income for both a pharmaceutical company and USA governmental agencies National Institutes of Health/Health and Human Services (NIH/HHS) that owned the technology used in the vaccine under the cover of doing something beneficial – a “greater good.” The HPV vaccine has less value than snake oil – at least snake oil is rich in Omega 3 EFAs, and consuming snake oil won’t harm anyone, but the same cannot be said for the HPV vaccine. The HPV vaccine was never necessary and the true interventions available for those who are concerned about preventing cervical cancer have been suppressed.
Does HPV cause cervical cancer?
While a virus may almost always be a trigger for cancer on a cellular/DNA level – it needs the environment, the internal milieu, to be prepared correctly to allow the wildcat cells to proliferate. Because the public doesn’t know this, it is easy for trusted authorities to impose fear of the virus on the populace just as was done and is still done with polio. It is a tried and true method of disinformation. In the case of cervical cancer the field upon which it takes hold must be deficient in certain vitamins, and without that deficiency it is very unlikely cervical cancer will take hold. While it may remain controversial which vitamins, or combinations thereof hold the key, the point is this is about a nutritional issue.
Indole-3-carbinol (I3C) is a phytochemical present in all members of the cruciferous vegetable family including cabbage, broccoli, Brussels sprouts, cauliflower, and kale. In a double-blind, placebo-controlled study,[1] 30 patients with biopsy-confirmed Cervical intraepithelial neoplasia (CIN), also known as cervical dysplasia, II-III were randomized to receive placebo or 200 or 400 mg oral I3C daily for 12 weeks. None of the patients in the placebo group had complete regression of CIN. In contrast, four of eight patients in the 200-mg/day group and four of nine in the 400-mg/day group had complete regression of CIN based on 12-week biopsy (400 mg/day, is equivalent to one-third of a head of cabbage.)
Adequate Vitamin D levels need to be present as well, but the point is cervical cancer is far more about malnutrition than an HPV virus. The vitamin D connection is no surprise because adequate vitamin D levels are required to have the immune system deal with viral infections and as most cancer patients are vitamin D deficient, regardless of what cancer they have a vitamin D/cervical cancer connection is a foregone conclusion.
Cigarette smoking, which is known to lower Vitamin D levels, only adds to the risk of cervical cancer. The bottom-line for women to understand is that an HPV infection alone is an insufficient cause of cervical cancer. HPV is but only one risk factor along with cigarette smoking. There is no direct link between HPV and cervical cancer. The vast majority of women will get an HPV infection but they will not get cervical cancer, but in malnourished women cervical cancer becomes a real risk. This is about poverty and nutrition – that is the direct link and the primary cause of cervical cancer.
Do HPV vaccines benefit women’s health?
Those who care about a woman’s risk of cervical cancer would do better to empower women everywhere and provide adequate nourishment, but this isn’t about caring about or for women – this is just about profit nothing less. Now, there has always been a pharmaceutical treatment for HPV infections (except in the USA). [2] One study showed that with just a ten day course of therapy with this extremely benign drug (inosine pranobex or Isoprinosine), there was an almost 80% elimination of human papillomavirus (HPV) 16 and 18 in cervical cancer (CIN I-III) and preinvasive cancer of the cervix and of those with recurrent CIN or Ca in situ in the remaining part of the cervix who were infected with (HPV).[3] This study was published the same year the FDA fast tracked the HPV vaccine.
The vaccines cover HPV 16 & 18, responsible for being the trigger for 70% of cervical cancers, but no one actually knows if the vaccine prevents infection (let alone cancer) – all we know is they increase antibodies to those two viral strains for an unspecified period of time. The vaccines do not cover 16 other HPV strains that can trigger genital cancer (31, 45, 33, 35, 39, 51, 52, 56, 58, 59, 26, 53, 66, 68, 73, 82). We do know getting the vaccine actually increases the risk of getting carcinoma in situ lesions from HPV strains not covered by the vaccine.[4]
Now this bears repeating, the FDA apparently knew the vaccine actually increased cervical cancer risk by 40% in women who had already been exposed to HPV and used magical thinking (no scientific evidence) to deal with this problem by recommending approving the vaccine for young girls hoping (I can only assume) they were never exposed – but the evidence is that infants can be exposed during the birth process, and since no testing of HPV serology is done before an HPV vaccine is given nor is it required, the FDA’s decision was irrational until you understand they were doing the bidding of not just Merck but the Health & Human Services Department that owns patents connected to this vaccine and would benefit if the vaccine became widely accepted.
Science or Subterfuge?
In other words, not only was the science behind this vaccine not there, but evidence showed cancer risk increased significantly. The fact that the NIH/HHS owned patents of the technology used in the vaccine, which was licensed to Merck, had everything to do with how this dangerous vaccine failed upward into approval and a fast-track. This is a total loss of boundaries between corporation and state.
This is now about criminal activity. When the head of the Merck vaccine division[5] (Julie Gerberding) is the same person in charge of the CDC you have an unholy alliance between corporation and state. The public and the world need to understand that no scientific information coming from the NIH/CDC/FDA/HHS can be trusted nor policies created from same. The loss of confidence in these agencies is irrecoverable.
Any scientific publication that is authored by anyone coming from or funded by someone either working for the government or a pharmaceutical company can no longer be trusted.
Whether or not the subterfuge behind HPV vaccines constitutes a violation of human rights deserves its own discussion. Nevertheless, several States (USA) will allow 12 year olds to receive this vaccine without parental consent. But a 12 year old cannot enter into a contract anywhere in the USA, so how could they possibly give informed consent for a vaccine?
Again, this aspect of the problem deserves international attention if not international legal intervention, but that is not taking place yet.
KP Stoller, MD* is President of the International Hyperbaric Medical Association, a lifetime Fellow of the American College of Hyperbaric Medicine, an Adjunct Assistant Clinical Professor (AT Still University SOMA), Chief of Hyperbaric Medicine – Amen Clinics, and was a Fellow of the American Academy of Pediatrics for over two decades until he resigned over their advocacy of mercury preservatives in vaccines. *these above organizations/institutions are for identification purposes only.
References available here:
http://sanevax.org/hpv-vaccines-human-rights-violation/
Notes:
[1] Bell MC, Crowley-Nowick P, Bradlow HL, et al. Placebo-controlled trial of indole-3-carbinol in the treatment of CIN. Gynecol Oncol. 2000;78:123-129.
[2] http://www.aig-journal.ru/en/archive/article/11092
[3]http://cat.inist.fr/?aModele=afficheN&cpsidt=18398598
[4] FDA’s VRBPAC Background document, used at the May 18, 2006 meeting where Gardasil approval was discussed:Page 13, Title: “Concerns Regarding Primary Endpoint Analyses among Subgroups, 1. Evaluation of the potential of Gardasil to enhance cervical disease in subjects who had evidence of persistent infection with vaccine-relevant HPV types prior to vaccination.” From
Page 14, Table 19, “Study 013: Analysis of efficacy against vaccine-relevant HPV types CIN 2/3 or worse among subjects who were PCR positive and/or seropositive for the relevant HPV type at day 1.” Table 19 shows that the efficacy rate for this group to be -33.7% ( A negative efficacy number means the vaccine led to an INCREASED risk of disease in the subgroups mentioned.)
Page 22, Table 32. “Detailed Safety Population: Number (%) of subjects who reported systemic adverse reactions of 2% or greater in the 15 days following receipt of study vaccine.” Table 32 shows that the number of subjects reporting systemic adverse reactions was 3591. That is a percentage of 59.2% of the participants.
[5] Technically, Gerberding did not become head of the Merck Vaccine Division until after she resigned from the CDC.
www.naturalblaze.com/2013/10/hpv-vaccines-human-rights-violation.html
More
- HPV Vaccines: A Human Rights Violati... befurther
12 y
7,722