Dateline NBC - February 20, 2011: A Case Study of Anecdotal Testimonies Masquerading as Scientific Journalism [Antineoplastons]
Last Updated on Tuesday, 26 April 2011 18:22
Click on the blue text within the article to view the sources used.
On February 20, 2011 Dateline NBC aired an entire episode based on Suzanne Somers' latest book"Knockout"—a book that featured a chapter on Dr. Burzynski's treatment and an entirely different cancer treatment offered by a New York physician named Nicholas Gonzales, MD.
The following essay focuses only on the Dr. Burzynski/Antineoplastons portion of the episode.
Introduction:
As the director and producer of "Burzynski, the Movie" I found a great interest in viewing this episode—particularly since I was asked to meet with Dateline NBC as a source while they were working on this special. I sat and watched my film in it's entirety with one of the lead producers on this special at "30 Rock", followed by a long conversation about the project afterward. She said "I can't say enough good things about you and the work you've done on this film Eric". As we ended our 3-hour meeting, I stated "in my time researching this subject, it's clear that the days of saying this treatment doesn't work are over, the jury is in." She replied "I want to do the right thing with this episode, but please remember that there is a good chance my bosses will not allow it." I also had numerous email communications with this producer and her assistant over the course of the next year.
I personally met with Dr. Nancy Snyderman when she and her Dateline crew attended the sold-out Newport Beach Film Festival screening of "Burzynski, the Movie" in the Spring of 2010 where they interviewed me on camera, and videotaped the entire hour-long Q&A session with Dr. Burzynski, Dr. Whitaker, myself and many cancer survivors who participated. I will not disclose this producer's name as I feel that she is not entirely responsible for the outcome of this episode.
Click here to read the 2/20/11 email newsletter that I sent out the afternoon before this special aired which expands upon my relationship with Dateline during the making of this episode.
You can view the entire Feb. 20, 2011 Dateline special, and follow along with this sourced essay, by clicking here and clicking on the "A Dose Of Controversy" link as shown below.
The analysis of this episode of Dateline is done in chronological order, and divided into three parts as outlined on NBC's website since the entire portion of this episode on Burzynski is broken up in to three parts.
Time codes for the sections discussed are notated:
Part One:
00:30-00:34: (Part 1)

Ms. Cassileth: "She's [Ms. Somers] not qualified to have on opinion on cancer treatment."
Response: This statement was made by Barrie R. Cassileth, Chief of Integrative Medicine Service at Sloan-Kettering. Ms. Cassileth is not a doctor. She holds a PhD in sociology [source: MSKCC]. Ms. Cassileth's opinion on this matter is also an unqualified one, as she too is not an oncologist.
00:40-00:47: (Part 1)
Dateline (Synderman): "While cancer patients of the unconventional doctors she promotes say they've been cured".
Response: These Antineoplaston patients aren't just saying they have been cured, they have been cured based on the official medical records of more than one doctor verifying that they have been cured. The only reason theybelieve they have been cured is because medical records prove that they have been cured.
Fact: Dateline reviewed all of the medical records of Dr. Burzynski's patients profiled in this episode verifying that they not only were officially diagnosed via biopsy or otherwise by outside "conventional hospitals" (in Jodi Fenton's case, a surgical biopsy) but they were also verified as "cured" by the very same standards and documentation as any "conventional hospital or physician " would judge such a cure. [source, patients & records]
00:52-00:57: (Part 1)
Dateline (Synderman): "So is Suzanne Somers selling real hope, or questionable medicine?"
Response: Why not look at the scientific data to see if she is providing "hope" or "questionable medicine"? You do not have to be a professional journalist or doctor to simply read the English language printed in the peer-reviewed scientific literature.
Is the National Cancer Institute and the American Cancer Society selling real hope, or questionable medicine? If we look at chemotherapy, based on a 14-year study, it has a mere 2.1% 5-year survival rate for all cancers combined [source, page 4 of PDF]. This isn't an opinion, it's just a scientifically factual conclusion based on the very same scientific method the "experts" are taught to respect.
00:57-1:05: (Part 1)
Dr. Andrew Weil: "She has put out a lot of misinformation, which I think can lead people to make not good decisions about their health."
Response: Dr. Weil is basing his only research on Dr. Burzynski by merely "visiting Burzynski's website" and not bothering to take the time to read the information on the website, review the FDA clinical trial data in the peer-reviewed medical literature, meet any patients, or review any scientific data on the subject.
On Dr. Weil's website, he states: "Over the years, Dr. Burzynski claims to have treated more than 8,000 patients, but his success rates are unknown. His web site states only that he has helped "many" people. If antineoplaston therapy works, we should have scientific studies showing what percentage of patients treated have survived and for how long, as well as evidence showing how Dr. Burzynski's method stacks up against conventional cancer treatment." [source: Weil's website]
Sadly, Dr. Weil never read any of the FDA-supervised clinical trial data which are "scientific studies showing what percentage of patients treated have survived" when he published this article about Burzynski. Therefore, Dr. Weil could be defined as someone who "puts out a lot of misinformation", based on the term's definition. Not to mention he fails to provide a single source for his article about Burzynski, leaving his unsuspecting readers to just "believe him". (He points to a Canadian study from 1985 where the majority of the patients died, a study which is highly suspect and is not printed anywhere in the peer-reviewed literature, ignoring all of the official and verifiable peer-reviewed FDA clinical trial data that have been ongoing since 1995—he pulled this from the American Cancer Society's website, who also lists no sources for this study—an organization that has nothing to gain andeverything to lose by reporting honestly on Antineoplastons).
00:57-1:05: (Part 1)
Dateline (Synderman): "Now, had anyone taken any biopsies of anything?"
Ms. Somers: "Not at this point."
Dateline (Synderman): "So a cancer diagnosis was made, but no tissue was taken anywhere?"
Ms. Somers: He said "you've got cancer, I've never seen so much cancer."
Dateline (Synderman): "And though she was told by her doctors that aggressive chemotherapy was her best hope, she decided against it. For five days she laid in the hospital, scared, grieving, preparing for the worst. The next day she says a biopsy proved the doctors wrong. She didn't have cancer."
Response: They first said she was in the hospital for "5 days", then continues to say "the next day a biopsy proved the doctors wrong". Which one is it? Even more frightening, was she offered chemotherapy before the biopsy? One would assume so—why would a doctor offer someone chemotherapy after a negative biopsy? Clearly they offered her chemotherapy before the biopsy—meaning that they were going to give chemotherapy unnecessarily to a patient who was never formally diagnosed with cancer at all.
Part Two:
00:13-00:20: (Part 2)
Dateline (Synderman): "... [the book] features several doctors which she claims can cure cancer without chemotherapy or radiation."
Response: "She" isn't "claiming" that Dr. Burzynski is curing cancer, the FDA clinical trial data printed in the peer-reviewed medical literature verifies that he is curing some cancers.
00:23-0025: (Part 2)
Dateline (Synderman): "So Dateline investigated, starting with the doctor she considers to be the most extraordinary."
Response: As you will find out by the end of this analysis, Dateline failed to investigate much of anything. They rely instead on anecdotal "expert testimony" from doctors who are not experts in the field of study they were asked to testify on behalf of.
00:35-00:45:
Dateline (Synderman): "Dr. Burzynski is considered something of a rock star of alternative cancer medicine."
Response: Wouldn't that be great if scientists in our society were given the same type of "rock star" status as Bon Jovi or Angelina Jolie? It's not like Bon Jovi or Angelina Jolie are curing cancer or anything. Isn't it scientists that have molded this world and made it a better place? Where would we be without the underpaid and under-recognized scientists and innovators of our world? Wright Brothers? Jonas Salk?
They then go on to show clips (shown below) from the sold-out Newport beach film festival screening of this documentary in April 2010, without mentioning the existence of the film—or mentioning that they have seen it and reviewed its sources. If they had mentioned the film, or mentioned that they sought my help in this Dateline episode based on my research while making the film—they would be forced to address the verifiable FDA clinical trial data and verifiable third-party medical records showing diagnosis and cures of the patients they profiled—among a mountain of other aspects of this story that run contrary to their "investigation" to make this episode—namely the peer-reviewed data on Antineoplastons printed in the medical literature.


00:50-00:59: (Part 2)
Dateline (Synderman): "as a young medical student in Poland in 1967, Dr. Burzynski says he discovered a group of proteins in blood and urine that cancer patients lack."
Response: Dr. Burzynski doesn't merely "say" this—it is verifiable in the scientific literature. Dateline again tries to give the illusion that scientifically verifiable evidence is merely anecdotal. [source: PubMed]
1:00-1:02: (Part 2)
Dateline (Synderman): He named them "Antineoplastons".
Response: This statement is perfectly accurate.
1:16-1:30: (Part 2)
Dateline (Synderman): "Dr. Burzynski treats the majority of his patients with conventional treatment.... thanks to Suzanne Somers book, he says a growing number are asking for his Antineoplastons which are not FDA-approved and considered experimental."
Response: "Treats the majority of his patients with tradiational treatment":
Dr. Burzynski treats the majority of his patients with FDA-approved gene-targeted therapies and does so in combinations that most oncologists are unaware of and are not traditionally taught. The follow up/part two of my documentary series on Dr. Burzynski will cover this method in great length. Dr. Bernadine Healy's article in US News and World Report describes exactly what Dr. Burzynski is doing in this respect, only without a computer and instead with the brains of a team of highly trained scientists and board-certified oncologists. This subject deserves and entire new effort, hence the reason for a new film about it. [source: Read "Breaking Cancer's Gene Code" by former NIH director Bernadine Healy, MD]
"...Antineoplastons which are not FDA-approved and considered experimental.":
All drugs within FDA clinical trials are considered experimental. That is the entire purpose of an "experimental FDA clinical trial". All drugs on the market today, without exception, had to pass through a series of FDA clinical trials, and were once considered "experimental" before approved for public use to be later considered "standard treatment".
1:31-1:51: (Part 2)
Dateline (Synderman): "Dr. Burzynski says most of the patients he sees have the worst kinds of cancer, and many are children, diagnosed with aggressive and inoperable brain tumors—for whom traditional treatment has failed. And yet he says he has patients still living 10, 15, even 25 years after getting his Antineoplastons."
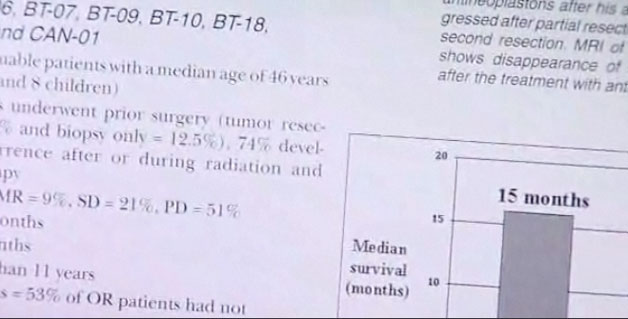
Response: Dateline continues to state what appears to be anecdotal evidence, instead of pointing to the peer-reviewed medical literature verifying this statement. Some childhood cancers, such as brainstem glioma, simply do not have a single drug approved by the FDA to treat it because not a single substance has ever shown any objective response on any patient with this condition—ever. Yet Dr. Burzynski has a 27.5% cure rate with these types of cancer vs. 0% with traditional treatment. Therefore, it's not that traditional treatment has failed these patients—but instead, there simply isn't a single medicine approved by the FDA in existence to treat patients with this condition at all. Radiation isn't a medicine, it's a carcinogenic radioactive treatment, and it has never shown toever cure an inoperable childhood brainstem glioma patient—ever. See sources of peer-reviewed FDA clinical trials comparing "traditional treatment" with Antineoplastons in childhood brainstem glioma patients: [Chemo/Rad - PubMed 2005] [ANP - PubMed 2003] [ANP - PubMed 2006] You can also visit childhood brainstem glioma survivor Jessica Ressel's page with full medical records and more clinical trial data here [source].
1:53-2:05: (Part 2)
They profiled Phillip Norton and his son Braiden Norton, who was diagnosed with a tumor in his brainstem:
Dateline (Synderman): "diagnosed by his doctors in Massachusetts with a tumor on his brainstem".
Response: Dateline acknowledged that Braiden's doctors in Massachusetts officially diagnosed his condition. Braiden was diagnosed with a pilocytic astrocytoma. Therefore, it would be irresponsible to state that Braiden was misdiagnosed. Yet, In part 3 of the special, Dr. Markman of MD Anderson tries to write off this patient as a misdiagnosis. Dateline fails to bring this up or revisit this mistake. [see Braiden Norton's website with medical scans before and after his Antineoplaston treatment].
2:10-2:33 (Part 2)
Dateline (Synderman): "..surgery removed most of the tumor, but not all of it, when it started to grow back doctors recommended chemotherapy, but Phillip Norton, a single father, didn't want to go that route." Synderman went on to ask Mr. Norton "what was it about chemo that didn't convince you?"
Mr. Norton: "It was never supposed to fix the problem guaranteed by any means."
(keep this in mind for later in this article)
2:45-2:52 (Part 2)
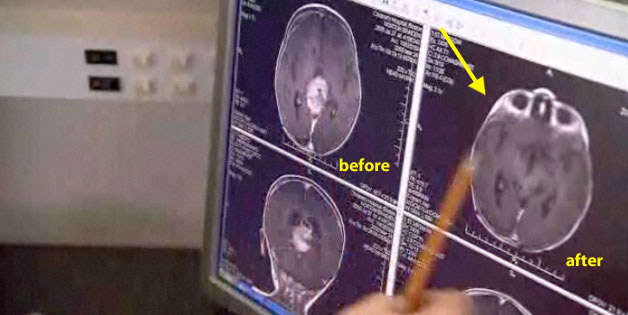
Dr. Burzynski: He [Braiden] started with us two years ago, and after one year the tumor is gone.
Dateline (Synderman): Is it gone or it is less?
Response: Synderman attempts to belittle and question the scans sitting in front of her. Look at the scan for yourself (below). What do you think? Is it gone or is is less?
2:53-3:03 (Part 2)
Dateline (Synderman): "Burzynski says he has hundreds of patients like Braiden, and adults too, like Jodi Fenton whose brain tumor was deemed inoperable in 2001."
Response: Incorrect. Jodi was diagnosed on May 15, 2000, not 2001. She was cancer-free within 30 days of starting treatment, and cancer-free in 2001 [source: see Jodi Fenton's story, medical records with biopsy from St. John's Medical Center in Los Angeles, FDA clinical trial data, and third-party confirmation by Oncoimaging in Herndon, VA confirming she was cured by Antineoplastons without any chemotherapy or radiation, as well as peer-reviewed medical literature covering her FDA clinical trial].

3:09-3:22 (Part 2)
Dateline (Synderman): "she says she refused the radiation her doctors recommended—choosing instead to be treated with Antineoplastons. Now Jodi, who says she's been cured, is expecting her second child."
Response: Jodi refused both radiation and chemotherapy. Dateline also fails to mention that Jodi was profiled in my documentary, with verifiable documented proof that she was cured solely with antineoplastons, with multiple third-party diagnosis including a biopsy performed and confirmed by Saint John's Medical Center in Los Angeles—and all of her medical records. They also fail to mention that if Jodi Fenton underwent the recommended chemotherapy and radiation offered to her—and had somehow survived, she would have most probably been infertile due to the treatment—unable to have any children at all. This apparently did not appear important to Dateline. Again, they based their "investigation" on what "she says" instead of referring the her medical records that were readily available to them–making the audience believe that her "cure" is based on Jodi's belief, not based on verifiable multiple third party medical records and FDA clinical trial data published within the peer-reviewed media literature.
3:29-3:39 (Part 2)
Dateline (Synderman): Yet, for over a decade the FDA and the Texas Medical Board doggedly but unsuccessfully pursued Dr. Burzynski an an attempt to shut his practice down.
Response: The majority of this documentary covers this 14-year legal battle in great detail for the majority of the film's running time. However, Dateline failed to mention that Burzynski was fighting the FDA and Texas Medical board while simultaneously cooperating with the FDA in Phase II clinical trials. An anomaly never before seen in history [source: Read a 12/5/1996 Washington Times article on this event]. They also failed to mention the eleven Antineoplaston patents that were being filed by one of Burzynski's own scientists in cooperation with Elan pharmaceuticals and the Department of Health And Human Services—the very same federal agency trying to indict Burzynski in court.
They also failed to mention that one of the reasons the Texas Medical Board failed to shut down Burzynski's practice is because the Founder of the Neuroradiology (brain tumor) Section of the National Cancer Institute, who is a board-certified radiologist, and professor of radiology at Georgetown University—Dr. Nicholas Patronas [source NCI]—testified as a brain cancer expert from our own National Cancer Institute in a Texas court in Burzynski's defense—stating "... it's amazing, the fact that they [Burzynski's brain tumor patients], the fact that they are living .... and they are not handicapped from the side effects of any treatment, and the side effects of the most aggressive previous treatment are worse than the tumor itself. So these particular individuals not only survived, but they didn't have major side effects. So I think it [Antineopalstons] is impressive and unbelievable." [source: Read the entire Texas Court testimony/transcript with Dr. Nicholas Patronas here].
3:58-5:05 (Part 2)
Dateline (Synderman): "Eventually, all of the charges were dropped except for one—for criminal contempt, and on that Dr. Burzynski was acquitted."
Response: The "final charge" was for supposedly shipping medicine across state lines illegally, which the second group of jurors (the FDA took Burzynski to court twice, after the first verdict wasn't to their liking) reviewed and ended in a "not-guilty" verdict. [New York Times 3/4/97 article] [New York Times 5/19/97 article] [You can also watch a clip of first half of the FDA legal section of the documentary here]
4:10-5:05 (Part 2)
Dateline (Synderman): "While his antineoplastons are still not FDA-approved, he can treat patients with them as long as they're enrolled in a clinical trial, which is how Braiden Norton got his... and he sees Dr. Burzynski's treatment as his son's best chance."
Response: Dateline alludes to Mr. Norton's perspective on Burzynski's treatment on his son as a "belief"—how he "sees" the treatment, vs. simply looking at the MRIs that show that the tumor is now gone, as well as the FDA clinical trial data published in the peer-reviewed medical literature showing that Antineoplastons have cured Braiden's type of tumor in the past.
Part Three:
00:00-00:13 (Part 3)
Dateline (Synderman): "Dr. Stanislaw Burzynski says he's curing pediatric brain tumors with his own brand of alternative treatment he calls Antineoplastons."
Response: Dr. Burzynski isn't "saying" anything, the FDA-authorized clinical trial data is verifying it.
00:18-00:27 (Part 3)
Dateline (Synderman): "You're the only man in the world who can save these kids?"
Dr. Burzynski: "Correct... not everybody, but some percentage of these patients can be saved."
Response: Dateline leads the viewer to believe Burzynski is not only claiming to be curing all types of pediatric brain tumors, but he is claiming that he is the only guy to do it. In reality, specifically in the case of childhood brainstem glioma—yes, he is the only one to do it in an FDA-authorized experimental clinical trial (sources listed earlier in this essay). He is not saying all types of childhood brain tumors, nor is he saying he can cure everypatient he sees.
00:58-1:08 (Part 3)
Dateline (Synderman): "Dr. Maurie Markman, one of the nation's leading cancer specialists, says there's never been definitive scientific evidence that antineoplastons have ever cured anyone."
Response: Dr. Markman is an oncologist from MD Anderson in Houston Texas, the same town that Burzynski's clinic is located [source]. MD Anderson has had a long-standing feud with Burznski's clinic due to many of MD Anderson's cancer patients fleeing MD Anderson to be treated by Burzynski, causing a loss in business by MD Anderson. One such patient was profiled in this documentary—Kelsey Hill. [Read her story, medical records and more here]. Secondly, once again, what can be more definitive than the results from FDA-authorized clinical trials of Antineoplastons showing clear percentages of survival in an established scientific setting? Again, if FDA clinical trial data published in the peer-reviewed literature is not definitive—we need to halt every single clinical trial being conducted for every single experimental medicine considered for FDA approval—right now—at this very moment—nationwide—immediately.

1:08-1:27 (Part 3)
Dateline (Synderman): "He says Burzynski's research has never withstood the scrutiny of other experts."
Dr. Markman: "If there's independent replication by other investigators, in other centers, that they see the same observation, then the public and future cancer patients can trust the observation."
Response: Dateline is asking the viewer to simply "take the word" of Dr. Markman's anecdotal testimony, and doesn't bother to research and verify if Dr. Markman's testimony is accurate. There are many other "independent investigators/scientists" around the world conducting "independent replication" of Antineoplaston studies—namely, independent researchers in Japan at Shandong University and Kurume University. [Shandong source 2007] [Kurume source 2005] [Kurume source 2003] [Kurume source 2002] [Kurume source 1998a] [Kurume source 1998b] [Kurume source 1996] [Kurume source 1995a] [Kurume source 1995b]. In Japan, Phase II trials have been completed, with Phase III trials underway for colon cancer metastasized to the liver (everyone is awaiting the final publication of these Phase III trials). Antineoplastons are set to be approved in Japan long before the United States, possibly resulting in a massive exodus of cancer patients into Japan upon their approval.
They also failed to mention that Phase III trials are the third and final phase of testing in the FDA-approval process. In the United States, Antineoplastons have successfully completed Phase II trials—which proves both safety and efficacy—and due to this accomplishment, Burzynski has been given permission by our FDA to begin Phase III trials, which consists of multiple independent facilities unrelated to Burzynski conducting the trials, which compare "standard treatment" (such as chemotherapy and radiation) to Antineoplaston treatment in two separate groups with the exact same type of cancers (known as the control groups and treated groups). These Phase III trials cost $150 million to complete, and since the NCI and federal government prohibit any taxpayer money (or cancer fundraising entities like "Susan G. Komen" or "Stand Up To Cancer") to help fund these trials, Burzynski is currently seeking funding on his own to pay for this. (Keep this in mind for later when Dateline addresses Burzynski over the cost if this treatment).
Also, Dr. Maurie Markman is a Gynecological Oncologist—not qualified to comment on brain cancer.
1:27-1:37 (Part 3)
Dateline (Synderman): "And when you see that—that doesn't happen, what goes through your mind?"
Dr. Markman: "There are lots of questions, the most important is 'well, perhaps, it's simply not true.' "
Response: We know, based on the verifiable evidence reported by Japanese researchers listed above, that there are "independent investigators/scientists" around the world conducting "independent replication" of Antineoplaston studies.
1:37-2:04 (Part 3)
Dateline (Synderman): "But Burzynski insists, he's been an open book, and published his data in replicated scientific journals for his peers to see. He gave me these journals he's published—it's proof, he's published."
Dr. Markman: "What these are, are abstracts presented in meetings, this is not peer-reviewed, this doesn't have any meaning in any way shape or form regarding the quality of the work or the ability to reproduce it."
Response: First of all, Dr. Markman picked up only the abstracts published in Neuro-Oncology, ignoring all of the other publications Dr. Synderman presented to him. Let's start with the Neuro-Oncology abstracts: Neuro-Oncology is a leading and very reputable brain cancer treatment related peer-reviewed medical journal. If these abstracts presented to Burzynski's peers at mainstream oncology meetings "have no meaning in any way shape or form regarding the quality of the work or the ability to reproduce it"—then why on earth does Neuro-Oncology bother publishing it? Can any random researcher go to one of these meetings and present some random work about hair-brained ideas on the effects of classical music sent through speakers surgically implanted into the brain next to the brain tumor, and then have Neuro-Oncology publish an abstract on that presentation? (Now, that would make a great documentary.) No. Neuro-Oncology would never publish such work if it wasn't verifiable. If they do publish such work that is unverifiable, you will see me in the next Oncology meeting presenting my own random ideas in hopes of getting them published in Neuro-Oncology. I think I will go with the "gold fish left lincoln logs in my sock drawer and that's what cured the brain tumors in these imaginary and unverifiable patients."
(A) This is where Dateline plays dirty: If you watch the episode from 1:39-1:40 they show an image of this document below:

This above image is a shot of none other than A PEER-REVIEWED ARTICLE PUBLISHED IN THE PEER-REVIEWED MEDICAL JOURNAL "DRUGS in R&D"
So, apparently Dateline showed their audience a shot of a peer-reviewed medical journal article drafted by Burzynski covering Multicentric Glioma while simultaneously stating that he has never published anything in a peer-reviewed medical journal.
For further confirmation that "Drugs in R&D" is a genuine peer-reviewed medical journal, see a press release about the journal stating "We reinvented Drugs in R&D as an open access journal to give researchers unrestricted access to all of its peer-reviewed content," said Anton van Rensburg, MSc, Editor, Drugs in R&D. "With increased pressure today for more speed and transparency in drug research and development, Drugs in R&D is an ideal outlet to further the cause by making its content publicly accessible and free for all." [source PR newswire]
So why did Dateline not want you to know about this peer-reviewed article? For two possible reasons:
#1 The peer-reviewed medical literature is the most sacred reference point to verify the truth behind scientific findings, as it has been shared with the "peers" of the scientific community for scrutiny, and upon proper scrutiny, this research is accepted for publication. If Dateline had acknowledged that this was done, the entire point of the episode to discredit Burzynski would be destroyed.
#2 On page 2 of this "peer-reviewed" article, it states the results of twelve children diagnosed with incurable recurrent and progressive multicentric glioma treated with Antineoplastons in Phase II clinical trials—six of whom were kids with pilocytic astrocytoma (the same type of tumor Braiden Norton is diagnosed with). All patients but one (due to dangerous location) had a biopsy to confirm diagnosis. RESULTS: Complete Response (cancer-free) was shown in 33% of the twelve patients with no progressive disease, with 25% with stable disease, and 25% with a partial response. That's 58% of the kids who were cancer-free or stable after Antineoplaston treatment. With the other 25% having a partial response. So, based on this peer-reviewed medical journal article published regarding this particular Phase II FDA clinical trial using Antineoplastons with pediatric brain tumors, 83% of these kids "responded". Ten of the twelve patients lived between 2 to 14 years (and counting) at the time of this publication.
This very same "peer-reviewed medical journal article" also cites comparisons to these types of pediatric brain tumors treated with "standard treatment". One group that treated 14 children with these types of tumors using radiation, chemotherapy, and lomustine—showed one patient (7%) had a complete response (cancer-free), four patients (29%) had a partial response, three patients (21%) had a stable disease, and six of them (43%) has a progressive disease (i.e. they died).
This very same "peer-reviewed" article also goes on to compare it's finding with another group of patients reported by The Children's Oncology Group using carboplatin with 50 patients. Two of them had partial responses (4%), 37 had stable disease (74%), and 11 had progressive disease (22%). None were cured.
So, Antineoplastons had a 33% complete response rate (cancer-free); radiation/chemotherapy/lomustine had a 7% complete response rate; and carboplatin has a 0% complete response rate. Printed right there in the "peer-reviewed" medical literature that Dateline panned across our screen. [source again: Phase II Study of Antineoplaston A10 and AS2-1 in children with recurrent and progressive Multicentric Glioma, Drugs R&D]
(B) But Dateline doesn't stop there, from time code 1:56-:1:59 they show this image:
This above image is from another "peer-reviewed" medical journal called "Integrative Cancer Therapies". For verification, their website states: "Integrative Cancer Therapies (ICT) is a peer-reviewed quarterly journalfocused on the scientific understanding of alternative medicine and traditional medicine therapies, and their responsible integration with conventional health care." [source]
This article explains in detail the gene-targeted mechanism behind Antineoplastons, with clinical data that destroys it's competition, right there for "his peers to see". [Read this peer-reviewed article for yourself here (Dateline's screen shot from page 8)]
No need to further embarrass the sloppy, amateur, high school-level anecdotal "journalistic" hacks known as Dateline any further with more breakdowns of the other two images of peer-reviewed literature they showed their audience while pretending it wasn't. If you would like to read more "peer-reviewed" medical journal articles regarding Antineoplaston clinical research that Dateline and Dr. Markman ignored (as well as the abstracts that "don't have any meaning in any way shape or form regarding the quality of the work or the ability to reproduce it") [go here]. While they are all a great read, this "peer-reviewed" report in the journal "Pediatric Drugs" is one of my favorites: [read here].
2:10-2:38 (Part 3)
Dateline (Synderman): "Let's say for instance I have a brain tumor, if I take these medicines, and seem to be stable, how do you know it's not the medicine in something else and vice versa.
Dr. Markman: "I don't know it's not the medicine, but I also don't know it is the medicine. It may very well be that small number that claim to do well, it may be again that that's the natural history of their cancers, they're slow growing. And of course there may be, uh, truly a misdiagnosis, someone said this person had a cancer, and they didn't—these are things we see all the time in routine practice."
Response: At least Dr. Markman admitted he hasn't any idea as to what on earth he's talking about—especially since he isn't a brain cancer specialist. It is the responsible thing to admit when one doesn't know an answer to a question within the field of science. That is what the scientific method is for—to test and re-test the idea and base a conclusion on those results. Or of course, take their knowledge-base from the "peer-reviewed medical literature". Dr. Markman does none of that. As clearly demonstrated in this article and the documentary film, all patients presented in this Dateline episode were verifiability diagnosed via biopsy or otherwise by third-party doctors—so to say these patients were never diagnosed is simply not true.
As far as brain tumors go, why don't we refer again to one of the most prominent brain tumor specialists in the country, Dr. Nicholas Patronas of the National Cancer Institute, who testified under oath that "I do not believe that spontaneous remission occurs [in brain tumors]; I don't think it does. And the available treatments only rarely produce results like that [Antineoplaston treatment]. The only medication—the only treatment, which I think is a last resort, is radiation therapy ... conventional chemotherapy is—provides very little—nothing, basically." [read this testimony for yourself, page 11]
Going back to "there may be truly a misdiagnosis, someone said this person had a cancer, and they didn't—these are things we see all the time in routine practice." Does this mean these "misdiagnosed patients they see all the time in routine practice" are undergoing chemotherapy and radiation unnecessarily?
3:01-3:08 (Part 3)
Ms. Somers: "Cancer treatment is a $200 billion a year business. Raising money for cancer research, where is the cure?"
Response: Feel free to check out Susan G. Komen's percentage of money that actually goes to "research".
Ms. Somers: "So, if some little guy in Texas is having success, what do they need him for?"
Response: The reality is, when or if this drug is FDA-approved for public use, The Burzynski Research Institute Inc. [BZYR] stands to corner the cancer treatment market with a 7-year exclusive license to make and sell the drug before it becomes generic. That is a big economic problem for PhRMA. Not to mention Burzynski has 2nd, 3rd, and 4th generation Antineoplastons that are much more developed and advanced than the ones in the current clinical trials. But, since he's stuck fighting a bunch of anecdotal testimony like this, an industry with 3 lobbyists for every one member of Congress trying to stop this type of competitive innovation, and an FDA bought and paid for by the pharmaceutical industry [source, Boston Globe], it's going to be a while before the public will be legally allowed to have these new advancements.
3:20-3:37 (Part 3)
Dateline (Synderman): "One person who isn't impressed is Dr. Barrie Cassileth, she's chief of the Integrative Medicines Service at Memorial Sloan-Kettering Cancer Center, and a long-time critic of Dr. Burzynski. She says his claims of success have gone too far.
Response: We have already covered Ms. Cassileth's lack of qualification to properly and responsibly be able to voice an objective opinion on Burzynski. She is a sociologist, and has no formal training in brain tumor oncology. Also, Barrie Cassileth has been commissioned to write and degrade all treatments considered to be "Unorthodox Cancer Medicines". [Read one of her reports here] [Here is another article Cassileth wrote concerning Burzynski, citing the botched NCI trials from the mid 1990's where the NCI themselves admit they diluted his medicine and ignored all of the agreed upon protocols in the only government-sponsored clinical trials of Antineoplastons they conducted—all published in their own literature (this event is covered at great length in the documentary with full sources)].
3:38-3:50 (Part 3)
Dateline (Synderman): "Dr. Burzynski showed me scans, pre-treatment and post-treatment brain scans, where he says 'look you can see there is no tumor there'. So is that not proof that some people are getting better?"
Cassileth: These are anecdotal reports.
Response: They are not anecdotal reports. They are patients treated within FDA-authorized clinical trials, and published within the peer-reviewed medical literature (any of this getting old yet?). Ms. Cassileth's testimony is the only thing anecdotal here. Again, if FDA-authorized clinical trials published in the peer-reviewed medical literature are merely anecdotal, again, we need to halt all clinical trials for all medicines nationwide, end all studies across the board and redesign the entire FDA-approval process.
4:05-4:13 (Part 3)
Cassileth: "How about the other people that see Burzynski and are dead? That is the context in which we have to understand these kind of anecdotes."
Response: How about the 97.9% of Stage IV cancer patients who choose chemotherapy that are also dead? [source: page 4 of PDF]. No one is claiming that all people entering an Antineoplaston FDA clinical trial will survive. No one is claiming this is a miracle cure at this stage. All we have to do is refer to the peer-reviewed medical literature to verify that it is not a miracle cure. However, referring to the same peer-reviewed medical literature reported from FDA clinical trials from 1995 up until today, you will not find a single cancer therapy that has come close to beating the percentages of long-term survival in the types of cancers Burzynski treats in FDA clinical trials with Antineoplastons [sources]. If any of you reading finds one, email me.
4:13-5:35 (Part 3)
Dateline then goes into a case of Halley Smith, a young girl diagnosed with an incurable (based on standard treatment) pediatric brain tumor. She is clearly one of the ones that do not survive, as the peer-reviewed medical literature on Antineoplastons has clearly reported.
Mr. & Mrs. Smith: "They wouldn't see us without money first".
Response: What about people like Robin Beaton who was denied her double mastectomy because her insurance wouldn't pay for it after "the hospital required a $30,000 deposit"? [source CNN] Did Robin get her surgery without paying for it first? No. Why should Burzynski be exempt from this basic practice of business? If insurance would simply cover this treatment we wouldn't have to listen to these sad stories. It is the fault of a corrupt system that forces the insurance companies to refuse covering Antineoplaston treatment that is to blame, not Burzynski. The cancer drug Avastin costs the patient's insurance company over $100,000 per year [source NY Times]—imagine trying to pay for that without health insurance. Avastin is only one drug, most people receiving Avastin also receive a whole slew of other insurance-covered medications.
5:37-5:48 (Part 3)
Dr. Markman: "Quite frankly, to go after vulnerable, helpless cancer patients with a claim that something will help, and have it never tested to prove it's benefit, and to profit, is to me the problem."
Response: As we have clearly established, it has been tested to prove it's benefit, and it's results are recorded in the peer-reviewed literature. As far as going after helpless cancer patients with a claim that something will help—and to profit, Dr. Markan just described the majority of all traditional FDA-approved cancer treatments on the market today, as well as himself.
6:00-6:10 (Part 3)
Dateline (Synderman): "How unusual is it to ask a cancer patient to pay up front?"
Cassileth: "I'm not familiar with that practice, within mainstream medicine."
Response: Ms. Cassileth is not familiar with this practice because she is a sociologist and does not deal with the billing side of being a cancer patient. Again, Robin Beaton is a fine example of a cancer patient who was forced to pay up front in mainstream medicine.[source CNN].
6:11-6:22 (Part 3)
Dateline (Synderman): "So Dr. Burzynski bills for other things, an amount his critics find unethical."
Response: I suppose this means everything should be free. All his board-certified oncologists should work for free, all his general medical doctors should work for free, all of his secretaries and other employees should work for free. Try telling that to Ms. Carrileth, Dr. Snyderman, Dateline's staff—tell them to work for free. How about Dateline's advertisers? (They should start placing any and all advertising on NBC for free—I will edit an advertisement tonight for this documentary to be placed on NBC during Dateline's next episode—for free). All of Sloan-Kettering's oncologists all work for free now!
The bottom line is that it's the fault of the medical industry that advises the insurance companies to not cover Antineoplaston treatment in any way shape or form, thus making the lives of Burzynski's staff and his patients more difficult. If we compare the cost of Antineoplaston treatment to most any other cancer treatment covered by insurance, you will find that Antineoplaston treatment is far less in cost. One would assume insurance companies would jump at this in a minute, until you realize that they are a part of the very same establishment that does not want this medicine approved for public use—due only to economic reasons. This medical discovery is bigger than the insurance companies, it's bigger than you and me, it has the potential to turn the cancer industry upside down financially. This is as big as the invention of the antibiotic, which by the way, also doesn't cure everyone.
6:56-7:00 (Part 3)
Dr. Burzynski: "This money should be reimbursed by the insurance companies..." (and they cut off the rest of his statement here).
Dateline (Synderman): "But insurance companies aren't always covering the costs."
Response: This was covered above.
7:19-7:30 (Part 3)
Dateline (Synderman): "But experts we spoke to disagree" [regarding Bariden Norton's brain tumor]. They said that the majority of brain tumor patients like Braiden survive, and that most of the tumors stop growing by adulthood."
Response: What experts? Where is their proof of this statement? Why is Dateline so afraid of backing anything they state with evidence instead of constantly carrying on with their anecdotal testimony that they masquerade around as science? According to the Children's Hospital of Boston, they find that "Inability to achieve a complete surgical removal [of a pilocytic astrocytoma] and the presence of recurrent disease decreases prognosis and long-term survival." [source]. I was unable to find anything anywhere in the peer-reviewed scientific literature that says these kids simply "outgrow them". You will also notice that Dateline doesn't show it's audience their "experts" stating this. For all we know some random sociologist told them this. And why does Dateline rely on these "experts" who are not experts in the fields they are asking them about at all—to inform them, why can't they find this data in the peer-reviewed literature themselves? One answer might be that most of what they stated can't be found in the peer-reviewed literature because what they stated is simply not true. Philip Norton emailed Dateline's producer (the same one I had been meeting with) to provide these "expert" sources. They refuse to answer this request.
Parts Four, Five and Six:
These sections are based on Dr. Nicholas Gonzales' treatment—unrelated to Antineoplastons.
Conclusion:
I had always known—intuitively—that the mainstream media is a marginalized entity incapable of distributing information that runs contrary to the status quo. This is particularly true in the categories of mainstream media that rely on advertising dollars for their survival. 99% of the advertising dollars given to the mainstream networks that air "the news" come from a market pool that replies entirely on profiting by upholding this very status quo. I have spent many years on Madison Avenue working for such advertising agencies as Young & Rubicam and the like. I myself have designed and launched campaigns for the pharmaceutical industry before I evolved a conscience. However, this Dateline episode has pretty much sealed my convictions concerning the mainstream media.
Knowing that I worked directly with Dateline, met with them in their main office at 30 Rockefeller Center in New York City, provided them with countless documents, invited them to attend and videotape the Newport Beach Film Festival screening (where the festival staff called this film "The Cove of 2011")—I sincerely never imagined them to stoop this low. However, I cannot blame them as I understand each of them want to keep their jobs. Nancy Snyderman is a major highly-paid television personality who would have been nailed to a cross had she reported honestly on this story. I get it, but it doesn't change my personal perspective on how the mainstream media not only intentionally lies to us, but they have to.
I have also worked on public relations campaigns in the past, the most recent being the promotion of this film. What most people do not realize is that when an entity like a pharmaceutical company sends out a press release, that press release hits the "news wires" and ends up in the studios and newsrooms of TV networks and newspapers. These news organizations generally simply "read" these press releases as if they are "the news" without having the slightest idea whether they are true or not. Almost never does the media question the press releases from PhRMA, the White House, or any member of the staus quo when they receive them. They just "read them" on air, or "print them" on paper.
Once fully educated on the subject, it's not hard to view all mainstream news organizations as highly suspect, and a waste of time. I suppose I should thank Dateline for giving me the opportunity to have a front row seat to witness first-hand the underbelly of an establishment that has zero integrity, one that is willing to prostitute itself to the masses as the shameless cogs in the wheels that help make it go round. Then again, that's the world we live in.



