J.Crow’s® Lugol’s Iodine
Free S&H.Restore lost reserves.J.CROW’S®Lugol’s Iodine Solut...
Energy Awareness Course
Use CureZone kode to get a free session!
Turmeric Bowel Cleanse
Hulda Clark Cleanses
J.Crow’s® Lugol’s Iodine
Free S&H.Restore lost reserves.J.CROW’S®Lugol’s Iodine Solut...
Energy Awareness Course
Use CureZone kode to get a free session!
Turmeric Bowel Cleanse
Hulda Clark Cleanses
Fullerenes for hydrogen storage
https://www.nanowerk.com/spotlight/spotid=2999.php
Posted: Oct 19, 2007
Fullerenes for hydrogen storage
(Nanowerk Spotlight) In our Spotlight on the issues of moving to hydrogen-powered cars (Nanotechnology could clean up the hydrogen car's dirty little secret) we briefly touched upon the problem of storing hydrogen onboard a vehicle. One gram of hydrogen gas will allow you to drive about 100 meters; unfortunately this single gram occupies almost 11 liters (2.9 gallons) of volume at room temperature and atmospheric pressure. In order to match today's cars' average reach of 400-500 kilometers per tank filling you would need to store 4 to 5 kg, or 40,000 to 50,000 liters, of hydrogen in your car. This is doable, but complicated and inconvenient, either by using intense pressure of several hundred atmospheres to store hydrogen as gas, or under cryogenic temperatures (minus 253 degrees centigrade) to store it in liquid form. Both alternatives have drawbacks. An intriguing nanotechnology approach to hydrogen storage is to encapsulate hydrogen inside hollow molecules, under room temperature. Fullerenes are ideal nanocages for this purpose, not only because they are hollow but also because hydrogen can be adsorbed on the fullerene surface. A new theoretical study provides the most accurate method to date for the structural optimization of such hydrogen-C60 composites, allowing to predict the hydrogen content in fullerene nanocages and their corresponding stability.
"We wanted to look at the properties of fullerene nanocages containing hydrogen and, as we studied the literature, found that the results in the few theoretical papers that considered endohedral C60 fullerene containing hydrogen molecules were contradictory" Dr. Boris I. Yakobson tells Nanowerk. "For instance, the maximum amount of hydrogen to form a stable hydrogen-C60 composite was determined as 23, 24, or 25 molecules. There even was a claim that there was not enough space for more than one hydrogen molecule inside C60. There is also a disagreement regarding the possibility of hydrogen chemisorption on the inner walls of the fullerene."
Since the previous studies did not agree with each other, Yakobson, a Professor in Materials Science and Computational Materials Science in the Department of Mechanical Engineering & Materials Science at Rice University in Houston, Texas, and his group decided to use a more accurate method – density functional theory – to determine the geometry of these composite structures, and to systematically study their properties for both high and low hydrogen content. They also performed ab initio molecular dynamics simulations in order to investigate the stability of the optimized structures.
The results of their study have been published in the October 9, 2007 online edition of Nano Letters ("Fullerene Nanocage Capacity for Hydrogen Storage").
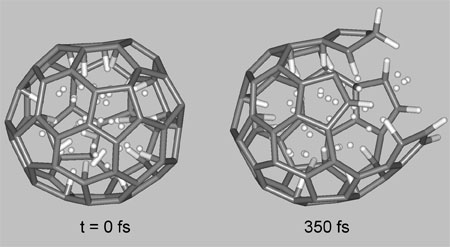
Ab initio molecular dynamics simulations of breaking C60 nanocage containing 58 hydrogen atoms at room temperature. (Image: Dr. Pupysheva)
"Most importantly" adds Olga V. Pupysheva, first author of the paper, "after investigating the hydrogen pressure inside C60, we find a general relation between the internal pressure and expansion of a fullerene nanocage of an arbitrary radius. This opens a way to make our results transferable, e.g., to giant fullerenes or nanotubes, that is, to predict the possible hydrogen content inside them without repeating ab initio calculations, which are overly expensive for such large systems."
Dr. Amir A. Farajian, a co-author of the paper, says that endohedral fullerenes (i.e. fullerenes encapsulating additional atoms) containing more than one hydrogen molecule have not been obtained experimentally yet. "It is very difficult to synthesize them, because they are highly endothermic" he says. "However, our calculations show that these structures can exist, and once they are created, they will not break easily. They have a high content of hydrogen, which can be released in a controllable way. So, if anybody manages to obtain them, they will be a great hydrogen storage media."
The Rice scientists also found that for a relatively small number of encapsulated hydrogen atoms – less than 20 – all the hydrogen inside C60 exists only in molecular form. If the fullerenes contain more than 30 hydrogen atoms, some of them form covalent bonds with the carbons of the fullerene cage.
Yakobson and his team determined that the maximum number of hydrogen atoms inside C60, which can form a metastable structure, i.e. corresponds to an energy minimum, is 58. Their model allows to calculate the maximum number of hydrogen that can be encapsulated in a stable fullerene of any given radius. For instance, a giant fullerene cage C720 could contain over 800 hydrogen atoms.
Another, maybe less practical but definitely not less interesting, achievement of this work is the demonstration of the exceptional mechanical strength of fullerenes.
"Just imagine" says Pupysheva, "they can keep so much hydrogen inside themselves, that the pressure is comparable with that inside the giant planets Jupiter or Saturn! Even though it is well-known that carbon nanostructures, such as fullerenes and nanotubes, are very strong, I myself was very surprised when I got these numbers."
While these calculations concerned the mechanical properties and stability of fullerene nanocages containing hydrogen, a significant hurdle for practical applications is to find a controlled mechanism to fill the fullerene nanocages with hydrogen and then release it when required. With the theoretical framework taking shape, the next main challenge is experimental – how to synthesize these structures, first in the lab and then for eventual mass production in commercial hydrogen storage tanks.
By Michael Berger – Michael is author of two books by the Royal Society of Chemistry: Nano-Society: Pushing the Boundaries of Technology and Nanotechnology: The Future is Tiny. Copyright © Nanowerk
This Forum message belongs to a larger discussion thread. See the complete thread below. You can reply to this message!
-
Fullerenes for hydrogen storage
 trapper/kcmo
6 y
267
trapper/kcmo
6 y
267
This is the position of the above message within the thread.
CureZone Newsletter is distributed in partnership with https://www.netatlantic.com
Contact Us - Advertise - Stats
0.109 sec, (5)

